Early on, C18 or C4 columns were the most commonly used chemistries for reversed phase chromatography. Recently, a variety of different chemistries, beads sizes, supports, and column configurations have become available making the choice of column more complex.
Chemistry:
The more hydrophobic the resin is, the stronger a hydrophobic column will bind it and it may become difficult to elute your protein of interest with a mild solvent. It is interesting to speculate how many prize-winning discoveries may be irreversibly attached to reversed phase columns in the drawers of labs around the world. For proteins without much prior knowledge, we recommend C4 matrices for polar proteins, and divinylbenzene matrices for those with more pronounced hydrophobic character.
Commonly found reversed phase chemistries:
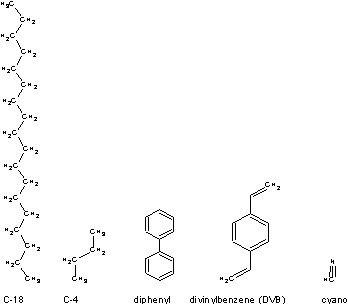
Bead Size:
The packing of a column and the accessibility of the substituents of the matrix are largely determined by the size of the beads which make up the resin. This, in turn, will determine the column to column variability, the capacity of the matrix, the amount of theoretical plates of a separation, and the backpressure the column generates. As a rule of thumb, smaller beads will give you a higher capacity column with higher pressures and more theoretical plates than a column composed of larger beads. For analytical and peptide work, 5 micron beads are often used, while for large scale purification of proteins and for low resolution desalting columns, bead sizes of up to 300 microns are available.
Supports:
The most common older support for matrices is the silanol bead with Si-OH groups being modified to attach the resin's ligand. These beads can be deprotonated at higher pH's and are destroyed by high pH. Mild deprotonation of the Si-OH groups will lead to a mixed chromatography: ionic interactions with the support and hydrophobic interactions with the ligand. This can lead to peak broadening and deterioration of your matrix. Thus, a common trick employed routinely with RPC is the use of a trifluoroacetic acid (TFA) counter-ion. This reduces the pH of your aqueous mobile phase to between 1 and 2, ensuring that the majority of the alcoholic groups are protonated and that the matrix is stabile. Additional effects of the ion pairing between trifluoroacetate and basic groups on the protein have been described to sharpen the peaks observed when running RPC and increasing the solubility of the protein upon lyophilization. A drawback of this aggressive pH is that some proteins do not retain structure or activity at the pH values described and the obvious drawbacks of use and disposal of an aggressive volatile acid such as TFA. Much more mild conditions can be used when choosing columns with polymeric support chemistries such as polystyrene which can be used at a wide range of pHs and is more likely to allow the protein of interest to retain its native structure during the RPC run.
Column Configurations:
Finally, one can chose from a variety of column configurations depending on the use of the purification, and the amount of material available. Smaller inner diameters make for high resolution separations with very little protein needed, while large columns may be required for industrial protein purifications. Capillary columns with inner diameters of 50 micrometers are routinely used for sub-nanomolar purifications of proteins especially for analysis by mass spectrometry. Microbore columns with inner diameters of 1 mm are useful for high resolution analytical work or small scale purifications when high resolution is needed with low sample amounts. Analytical columns with diameters of up to 5 mm are every day use RPC columns for routine analytical and purification work. Preparative columns are larger in diameter and can be used for purification of large quantities of proteins in the mg to gram scale.